Introduction
Clinical outcomes in newly diagnosed multiple myeloma (MM) are independently influenced by genetic risk as determined by FISH and molecular profiling. However, little is known about the impact of these characteristics on HRQoL of MM patients at diagnosis, and if this is independent of other diagnostic criteria such as International Staging System (ISS) and CRAB. Similarly, there is little quantitative information on HRQoL of MM patients in contrast to other cancer patients and healthy individuals. We examined patient-reported outcomes from participants enrolling in the RADAR study to understand the HRQoL of MM patients in different subgroups prior to treatment.
Methods
The RADAR study (Eudract: 2019-001258-25) is a national, multi-centre, risk-adapted, response-guided multi-arm, multi-stage phase II/III trial for newly diagnosed MM eligible for ASCT. RADAR treats patients with induction, consolidation and maintenance options using combinations of lenalidomide, cyclophosphamide, bortezomib, dexamethasone and isatuximab.
Genetic risk was defined using centrally reviewed standard-of-care NHS testing and participants enter the standard or high-risk pathway based on the presence of at least two of the markers: t(4;14), t(14;16), t(14:20), del(17p), gain(1q) and del(1p). CRAB diagnostic criteria at baseline were defined as hypercalcaemia (>2.6 mmol/l), renal impairment as indicated by eGFR by MDRD (30-60, >60 mL/min/1.73m 2), anaemia (Hb<100g/L) and the number of lytic lesions at trial entry (0, >=1).
HRQoL was measured by EORTC QLQ-C30 and MY20 completed at trial entry. We used one-way ANOVA and two-sample unequal variance t-tests to compare subscales in standard and high-risk groups and CRAB criteria groups. We sought minimally important differences that were at least medium by accepted definitions, and adjusted p-values using the Bonferonni correction for the 19 subscales (adjusted P = 0.05/19 = 0.0026). We undertook similar comparisons with normative scores (NORM) for cancer patients and UK healthy controls.
Results
Baseline HR-QoL was available for 301 participants enrolled in RADAR. Median age at trial entry was 61 (range, 34-74), 165 (57.5%) were male and 249 (88.3%) ECOG PS0-1.
235 (78.1%) patients were classified as standard risk and 52 (21.9%) as high-risk. 111 (38.7%) patients were ISS1, 116 (40.4%) were ISS1 and 50 (17.4%) were ISS3. 24 (8.6%) patients had hypercalcaemia, 48 (16.7%) patients had eGFR < 60 mL/min/1.73m 2, 67 patients (23.8%) had anaemia and 287 (65.9%) patients had at least one bone lesion.
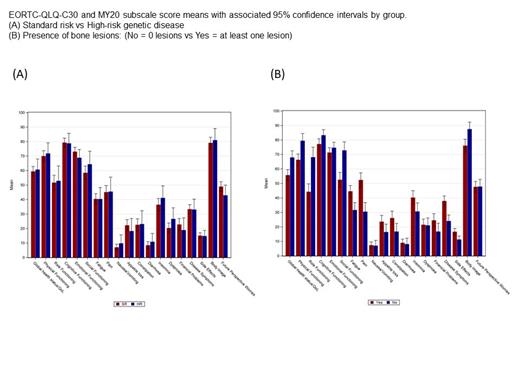
Comparing standard and high-risk genetics patients, there were no significant differences in any C30 or MY20 subscales (Figure A). Increasing ISS was significantly associated with worse Physical and Role Functioning, increased Fatigue and Dyspnoea. Renal impairment (eGFR< 60 mL/min/1.73m 2) was not associated with differences in any C30 or MY20 subscales.
Anaemia was significantly associated with worse Global health status/QoL, Physical Functioning and Role Functioning, and Fatigue was significantly increased. The presence of bone lesions was significantly associated with worse Global health status/QoL, Physical Functioning, Role Functioning and Social Functioning, and Fatigue, Pain, Disease Symptoms, Side Effects and Body Image worries were significantly increased (Figure B).
Comparing RADAR to the C30 Cancer NORM, 7 of 15 subscales were significantly worse. Small negative differences were observed for Physical and Role Functioning, Fatigue, Insomnia and Constipation and medium negative differences were observed for Social Functioning and Pain. Similarly, in contrast to UK C30 Healthy NORM, 8 of 15 subscales were significantly different. There were small detrimental differences observed for Physical Functioning, Role Functioning, Appetite Loss, Constipation and Financial Problems, medium differences for Fatigue and Pain and large differences for Social Functioning.
Conclusion
Prior to commencing treatment, patients with genetic high-risk MM did not have significantly different HRQoL to standard risk MM whereas ISS, an established measure of disease burden, did impact HRQoL. Similarly, CRAB criteria of anaemia and bone disease significantly impact several HRQoL domains.
Compared to the general Cancer and UK healthy population HRQoL in patients entering RADAR was significantly worse in some subscales, notably Pain and Fatigue.
Disclosures
Chapman:Celgene (BMS): Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria. Cook:Sanofi: Consultancy; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Amgen: Consultancy; Karyopharma: Consultancy. Jackson:Celgene BMS: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; J&J: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Speakers Bureau; Oncopeptides: Consultancy. Jenner:Janssen, BMS, Pfizer, Sanofi: Consultancy, Honoraria. Kaiser:Regeneron: Consultancy; Pfizer: Consultancy; GSK: Consultancy; Janssen: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Seagen: Consultancy; Karyopharm: Consultancy. Popat:GSK: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria. Smith:Janssen, Abbvie, Takeda, BMS, Menarini, Sanofi: Honoraria, Other: Support for conference travel and attendance. The research funding referred to above is agreed inprinciple but has not yet been received and may not be until 2024., Patents & Royalties: Honoraria for speaking, assiting in training events, Research Funding. Ramasamy:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS ( Celgene): Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Recordati: Honoraria; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Menarini Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cairns:Takeda: Research Funding; Janssen: Honoraria; Celgene BMS: Honoraria, Research Funding; Amgen: Research Funding; Sanofi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal